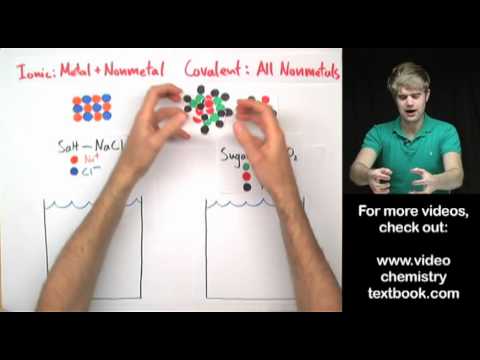
What happens when stuff dissolves? I mean, what happens to the atoms and molecules then? Let's talk about two things: salt and sugar. - These two substances look very similar. They are both white powders made up of little grains. - You can pour both salt and sugar into water and stir them around. As you do, you will see the grains getting smaller and maybe breaking apart until they totally disappear. - This is what happens with the naked eye. But what if we could see the atoms that make up salt and sugar? - Let's explore how they behave when they dissolve. - The behavior depends on whether something is an ionic or a covalent compound. - Salt is an ionic compound made of sodium ions (Na+) and chloride ions (Cl-). - Sugar, on the other hand, is a covalent compound made of nonmetals: carbon, oxygen, and hydrogen. - When we dissolve salt, the atoms that make it up come apart and float around individually in the water. - When we dissolve sugar, the molecules break up, but they remain as individual molecules, not individual atoms. - It is a common misconception that covalent compounds like sugar break apart into individual atoms when dissolved, but this is not the case. - So, the short answer to what it looks like when something dissolves in water is that it depends on whether it is an ionic or a covalent compound. - Ionic compounds break apart into individual atoms, while covalent compounds break apart into individual molecules. - In both cases, the substances dissolve and become part of the water.
Award-winning PDF software





Video instructions and help with filling out and completing Are 8850 Form Solutions