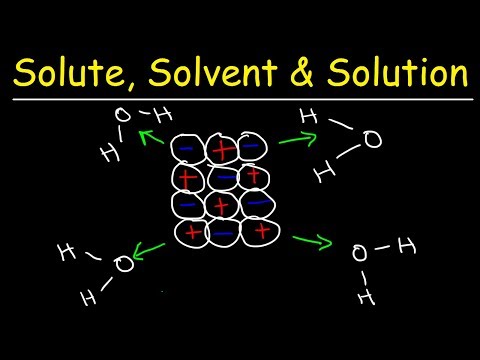
Here is the corrected version: So, what does it mean when a compound is soluble in water? What happens when a compound is dissolved in water? Let's use sodium chloride as an example. Sodium chloride is table salt. If you were to pour table salt into water, the salt would appear to disappear. It would dissolve in the water, and you won't see it anymore. You would just have a clear solution. But what's actually happening inside? In sodium chloride, you have positive sodium cations and negative chloride ions attached to each other. So, you have this big solid that's composed of ions. Once you place this ionic crystal in water, the water molecules are going to pull apart this crystal. Water is polar. The oxygen part of water has a partial negative charge, and the hydrogen part is partially positive. Overall, water is electrically neutral, but oxygen is more electronegative than hydrogen, so it pulls the electrons toward itself, giving oxygen a partial negative charge and hydrogen a partial positive charge. Due to this partial charge of the elements in water, it becomes polar. And many salts, like sodium chloride and other compounds, tend to dissolve in polar solvents. The oxygen part of water is attracted to sodium. Now we can see why opposites attract. Sodium has a positive charge, while oxygen has a partial negative charge. The hydrogen part of water, which has a partial positive charge, is attracted to the negatively charged chlorine atom. So, what happens is that all of these water molecules are slowly pulling each ion in the crystal away from each other. The oxygen is pulling away the sodium ions, and the hydrogen atoms are pulling away the chloride ions. Eventually, these ions move apart and become more attracted to water than to themselves....
Award-winning PDF software





Video instructions and help with filling out and completing Where 8850 Form Solutions